LDF forces must also be considered in determining the relative. So the boiling point of D2O is higher than H2O not because of the London forces but because of the difference in electronegativity between the hydrogen and the water creating an electrostatic attraction.
10 6b Trends In Boiling Points Melting Points And Enthalpies Of Vaporization For P Block Binary Hydrides Chemistry Libretexts
Referring to both species in.
. Boiling points and melting points differ for different elements and compounds. This is because HF is able to form Hydrogen bonds whereas the other three molecules are unable to do so because their electronegativity is not large enough to create a sufficient dipole. Let us look at the elements in the ascending.
The melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. Explain the trend in the boiling points in terms of bonding. There are 3 important trends to consider.
The boiling point then increases down the group from HCl to HI because the halogens increase in size. Branching decreases boiling point. Ionic Hydrogen bonding dipole dipole Van der Waals dispersion forces.
When a substance melts some of the attractive forces between particles are broken or loosened. Which essentially implies breaking a few bonds. The graph shows how melting points and boiling points vary down group 2.
Yeah Water molecules have Van der Waals interactions as well as hydrogen bonds hence it requires more energy to break the bonds higher boiling point just done this in Chemistry today. Intermolecular Forces and Trends in Boiling Points Trends in boiling points are not necessarily straightforward. So more energy required to remove 3rd electron in Mg than in F as outer electrons more strongly attracted by.
This begs the more interesting question. The hydroxyl group of 1-pentanol is more exposed than it is in 3-pentanol which is flanked by two bulky alkyl groups so it will be better. Thus higher the stronger the bond between the atoms higher will be the melting point.
Boiling of a liquid involves increasing the. Melting and boiling points of group 2 elements Be Mg Ca Sr Ba Ra Group 2 element 0 500 1000 1500 2000 2500 3000 Temperature K. Hydrogen- bonding occurs when hydrogen is bound to a STRONGLY electronegative element ie.
Water molecules are strongly attracted to each other via what are called hydrogen bonds. Explain why the boiling point of water is higher than would be expected from the group trend. JavaScript chart by.
JavaScript chart by amCharts 32115. 3 Trends That Affect Boiling Points Master Organic Chemistry 4162021 50314 PM It can also apply to hydrogen bonding molecules like alcohols compare the boiling points of 1-pentanol to 2-pentanol and 3-pentanol for instance. You can rationalize or explain these differences by thinking about the structure of each molecule.
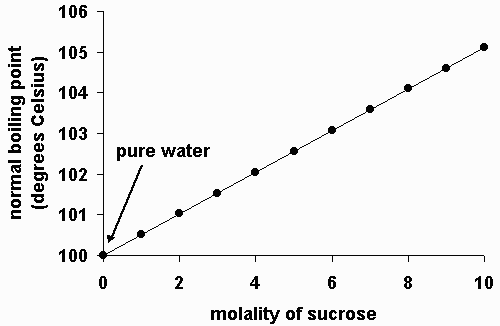
Pure water has a fixed boiling point at standard atmospheric pressure. Boiling points increase as the number of carbons is increased. Ionic Hydrogen bonding dipole dipole Van der Waals dispersion forces.
The particles can move around each other but are still close together. Common table salt sodium chloride the boiling point of the water changes. Answer 1 of 4.
The relative strength of the four intermolecular forces is. Clearly there is an intermolecular force operating between the water and ammonia molecules the which you have already identified. However if water is considered the freezing point is 0.
Explain the trend in boiling points from H2S to H2Te. Ethanol for example boils at a lower temperature than water. H-Cl H-Br H-I All three molecules are polar so relative dipole forces will important.
Why is deuteriums electronegativity different. However if some impurities are added in the water eg. The relative strength of the four intermolecular forces is.
The boiling point of a substance is the temperature at which the vapour pressure of the substance becomes equal to the ambient pressure and bubbles of vapour form directly in the liquid. When a substance boils most of the remaining attractive forces are broken. The influence of each of these attractive forces will depend on the functional groups present.
Van der WaalsLondon dispersion forces increase ii. At another extreme in Death Valley Calif. Yes H2O is a polar molecule and as a result it forms hydrogen bonds with other water molecules.
2 Explain why the boiling point of water is higher than would be expected from the group trend. The particles can move freely and are far apart. The boiling points of the hydrides of the group 6 elements are shown below 1 Explain the trend in boiling points from H 2 S to H 2 Te.
The influence of each of these attractive forces will depend on the functional groups present. Explain why the boiling point of water is higher than would be expected from the group trend. It takes more kinetic energy or a higher temperature to break the hydrogen bonding between water molecules thus allowing them to escape as steam.
Water has a high boiling point because its molecules are bound together by hydrogen bonding which is a very strong intermolecular force. This means that the van der waal forces are stronger down. The water molecule has an.
Magnesium is excluded to begin with but you can toggle it on and off. P what a coincidence. Boiling points increase as the number of carbons is increased.
The lower atmospheric pressures at high elevations affect cooking and baking too which is why many recipes and mixes come with special high altitude directions. For H2S H2Se and H2Te as sizemassMr increases van der Waals. If 100 grams of salt is added to 1 kilogram of water the boiling point of the impure water would increase by 1 degree Celsius.
For example which of the following substances has the highest boiling point. Propane is a hydrocarbon and a gas while gasoline a mixture of hydrocarbons is a liquid at the same temperature. Explain why the H-N-H bond angle of NH3 is different from the H-N-H bond angle of NH4.
An oxygen from a neighboring water molecule attracts the hydrogens in this molec. The lowest point in the US at 282 feet below sea level water boils at slightly above 212 degrees. The table below gives a brief summary of these sections.
The normal boiling point is specified when the ambient pressure and thus also the vapour pressure of the BOILING LIQUID is 1atm. H-Bonds strengthen the bonds between. In the process youll gain some new insights into everyday chemistry.
The water molecule is H-O-H with oxygen pulling negatively charged electrons closer to it than the hydrogens. I as molecules become largerheavierhave higher M r values number of electrons increases. Nitrogen or oxygen or fluorine and in fact we could recognize that the boiling point of H F 195 C is ALSO rather.

3 Trends That Affect Boiling Points Master Organic Chemistry
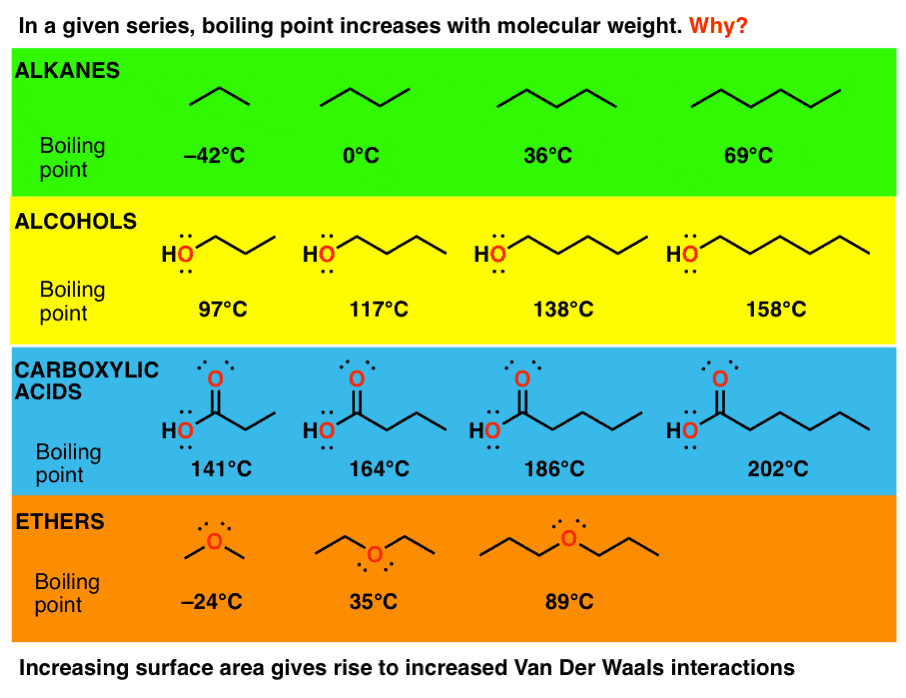
3 Trends That Affect Boiling Points Master Organic Chemistry
Why Does The Boiling Point Of A Homologous Series Increase As Molecular Mass Increases Quora
0 Comments